Glycogene Continues to Make Breakthroughs: Various Phosphoramidite Monomer Products Receive FDA DMF Filings!
Release time:
2024-09-06
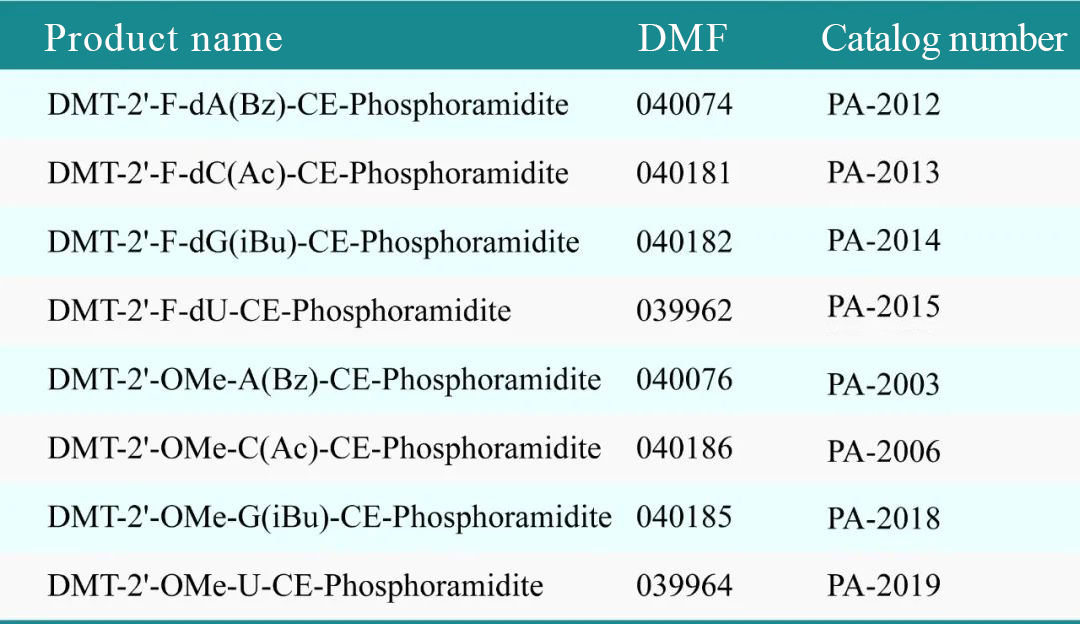
Summary:Recently, Glycogene received a confirmation letter from the U.S. Food and Drug Administration (FDA) stating that all 8 phosphoramidite monomer products submitted by the company have officially completed the Drug Master File (DMF) filing with the FDA. This marks the first time globally that phosphoramidite monomer products have completed DMF filing with the FDA. The 8 products submitted are part of the 2'-O-Me and 2'-F series, which are frequently used in small nucleic acid drugs.

Recently, Glycogene received a confirmation letter from the U.S. Food and Drug Administration (FDA) stating that all 8 phosphoramidite monomer products submitted by the company have officially completed the Drug Master File (DMF) filing with the FDA. This marks the first time globally that phosphoramidite monomer products have completed DMF filing with the FDA. The 8 products submitted are part of the 2'-O-Me and 2'-F series, which are frequently used in small nucleic acid drugs.
Drug Master File (DMF) is a document submitted by pharmaceutical companies to regulatory authorities (such as the U.S. FDA) to provide confidential information about drug substances, intermediates, packaging materials, and other related components. It typically includes Chemistry, Manufacturing, and Controls (CMC) data, stability data, packaging material data, and other relevant information to verify the quality, safety, and efficacy of the product.
After completing the DMF filing, when you use Glycogene DMF-filed products for FDA submissions, you can directly reference the corresponding DMF number instead of providing detailed information about the raw materials and excipients during the submission process. This not only reduces approval costs but also enhances approval efficiency, helping to accelerate the regulatory submission process for your related drug projects.
Product Advantages
1. GMP Compliance
-
The products are manufactured under a GMP (Good Manufacturing Practice) quality management system, ensuring the controllability and traceability of materials and processes. We are fully prepared to support customer on-site audits at any time.
2. High Purity
-
The product purity exceeds 99%, with strict control over moisture, residual solvents, and other impurities.
3. Large-Scale Production
-
Annual production capacity reaches ton-scale per product.
4. Self-Production
-
Core raw materials, such as the 2-F nucleoside series, are produced in-house, ensuring quality and supply chain stability.
Working Together, Chasing the Light | Tangzhi Pharmaceutical 2025 New Year Gala
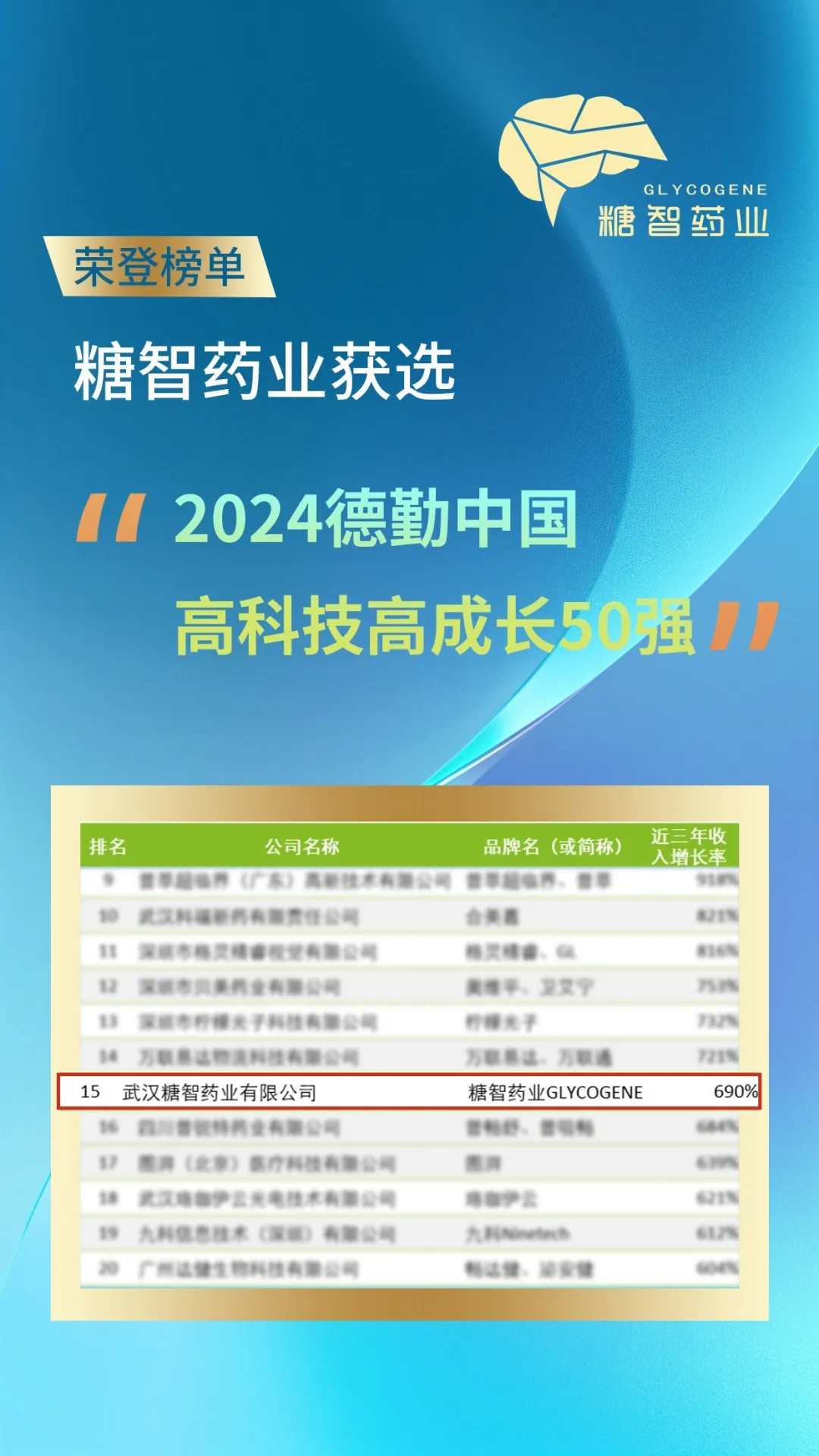
Summary: As the year turns and a new chapter unfolds, we look back on 2024, a year of progress and achievement for Tangzhi. This year, we held grand annual meetings in Wuhan, Qingdao, and Panjin, celebrating our successes together. Wuhan, as the headquarters of Tangzhi Pharmaceutical, holds our initial aspirations and dreams. In 2024, we achieved remarkable results. The company was successfully selected for Wuhan's "Backbone High-tech Enterprise Gazelle Plan" and recognized as a "Potential Unicorn" enterprise in the East Lake High-tech Development Zone, a "2024 Deloitte China High-Tech High-Growth 50 Strong" company, and a "2024 Deloitte Guanggu High-Tech High-Growth Top 20" company. Over the past year, Tangzhi Pharmaceutical has continued to focus on technological innovation and product development, resulting in steady revenue growth. At the Wuhan annual meeting, in his opening address, Chairman Wang Peng reviewed the outstanding achievements we made in technological innovation and market expansion, thanked every employee for their hard work, and looked forward to the new year, where we will continue to cultivate the fields of sugar and nucleic acids, providing even better products and services to global customers.
2025-01-27
Summary: Recently, the "2024 Deloitte China Technology Fast 50" (hereinafter referred to as "China 50") was announced. Tangzhi Pharmaceutical, with its outstanding performance and rapid growth in the fields of sugar and nucleic acids, was honored to be on the list, ranking 15th with a revenue growth rate exceeding 690%! This honor is not only an affirmation of Tangzhi Pharmaceutical's innovative strength in the fields of sugar and nucleic acids, but also a commendation for its high-speed growth in a difficult environment. The Deloitte China Technology Fast 50 program has always been regarded as a "benchmark for high-growth companies globally." The selection of Tangzhi Pharmaceutical marks its leading position and strong competitiveness in the industry.
2025-01-21
New DNA-encoded chemical library technology to aid drug discovery and development
Summary: (Science and Technology Daily) Although researchers have made significant progress in the development of molecular therapies in recent years, the number of newly discovered active substances remains insufficient. Now, the DNA-encoded chemical library (DEL) technology, jointly developed by Harvard University in the US and ETH Zurich in Switzerland, offers a new solution. The technology can automate the synthesis and testing of billions of compounds in a matter of weeks, and can also be used to produce larger drug molecules that will act on targets that are difficult to reach with traditional small molecules.
2024-09-25