Glycogene Phosphoramidite Monomer Product Receives FDA DMF Filing
Recently, Glycogene received a confirmation letter from the U.S. Food and Drug Administration (FDA) stating that the company's submitted products, 5'-O-DMT-2'-O-methyluridine 3'-CE-Phosphoramidite (Um) and 5'-O-DMT-2'-deoxy-2'-fluorouridine-3'-CE Phosphoramidite (Uf), have officially completed the Drug Master File (DMF) filing with the FDA. This marks the first time globally that phosphoramidite monomer products have completed DMF filing with the FDA.
Jul 02,2024
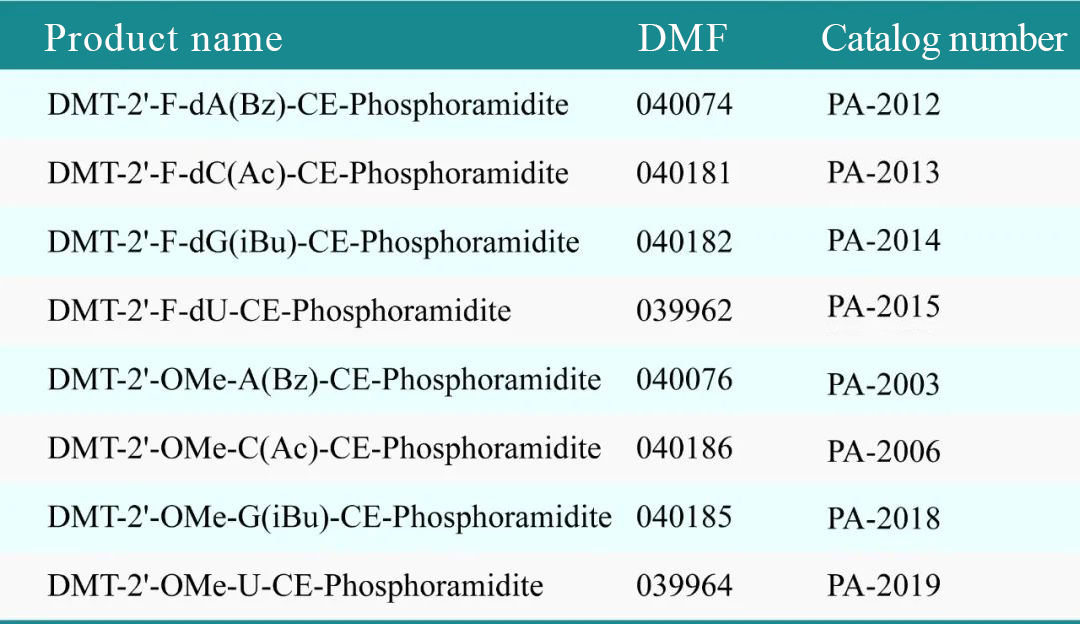
Recently, Glycogene received a confirmation letter from the U.S. Food and Drug Administration (FDA) stating that all 8 phosphoramidite monomer products submitted by the company have officially completed the Drug Master File (DMF) filing with the FDA. This marks the first time globally that phosphoramidite monomer products have completed DMF filing with the FDA. The 8 products submitted are part of the 2'-O-Me and 2'-F series, which are frequently used in small nucleic acid drugs.
Sep 06,2024
RELATED INFORMATION